The Simple Answer #
Volcanic minerals (rocks) created under pressure in the earth, when brought to the surface are somewhat unstable. When these magnesium and calcium silicate rocks are exposed at the surface of the earth and come into contact with water and carbon dioxide (CO2), such as CO2-dissolved-in-rainwater (carbonic acid) during normal rainfall, a reaction known as “chemical weathering” occurs, which causes the mineral to break down, releasing its constituent parts, including magnesium and calcium particles (cations).
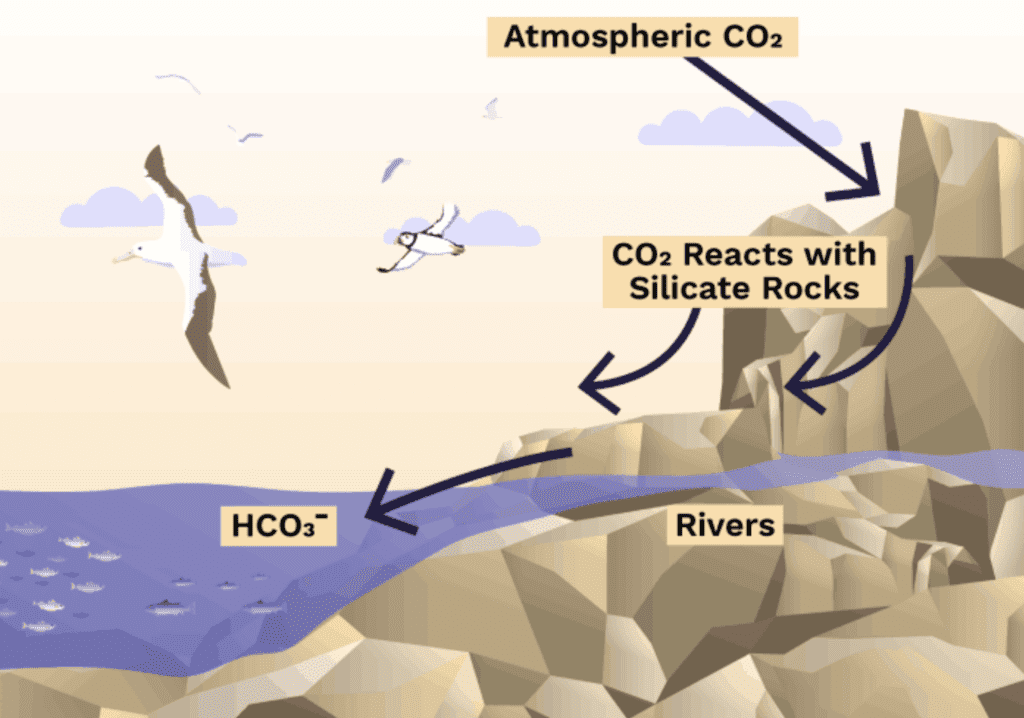
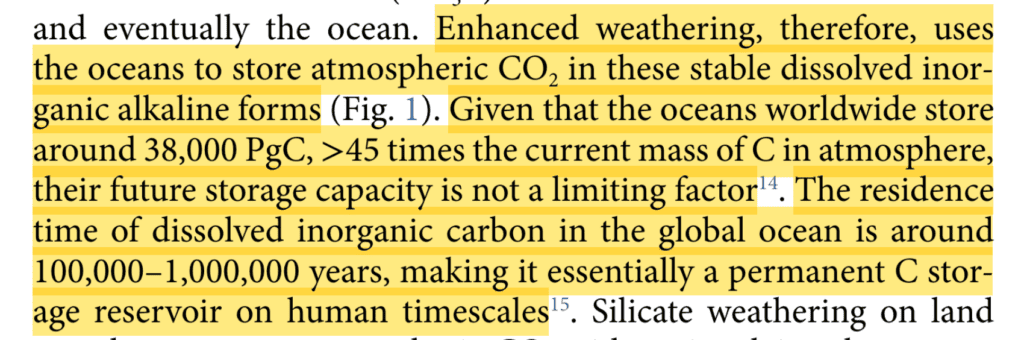
These cations are positively charged particles Mg++ (Mg2+) or Ca++. These positively charged particles interact with carbon dioxide and cause it to form bicarbonate (HCO3–), which contributes alkalinity to the system. As the rain run off eventually makes it to the ocean, most of the carbon dioxide dissolved will remain dissolved in seawater as alkaline, bicarbonate for 10,000s-100,000s years.
When you see mineral water advertising a high pH, that high pH comes from the minerals dissolved in it from the aquifer it came from (or were added). If you check the label you will typically see the minerals listed as well as bicarbonate. You can think of ERW as a method to generate the molecules that lead to the storage and therefore “removal” of carbon dioxide in the ocean.

If you were to take your expensive alkaline mineral water bottle with magnesium cations and pour it into the ocean, it technically does lead to some small amount of carbon dioxide removal. What we are doing is essentially creating a very large amount of alkaline water and safely using rivers to transport it over time to the ocean, but speeding up the natural process by thousands to million of years.
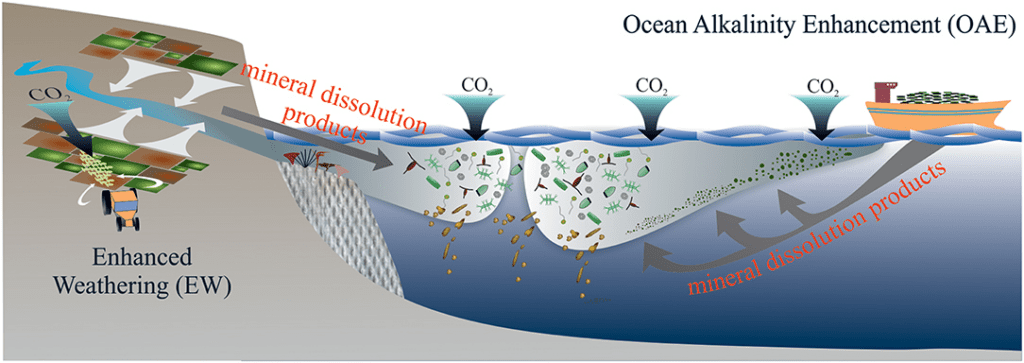
Soils are a natural place to speed up weathering because they have much CO2 concentrations more than 3 times the ambient air and soil an and bring the CO2 in contact with water in soil pore. which drives weathering, along with the ability to add water at will. Because the process of ERW is spread out over land and time, and due to the utilization of riverine transport in ultramafic areas, we avoid overly large spikes at any one time.
As I will describe below about the long-term carbonate silicate cycle of which this process is a part, it generally takes tectonic forces and the chance for these types of rocks to be exposed in the tropics for enough mineral weathering to occur to reduce global temperatures. However, in times when this has occurred by chance, it lead to global cooling. Unfortunately, we do not have time, on human time scales, to leave this up to chance any longer.
The Complicated Answer #
The more complicated answer of how CDR with ERW works in the ocean is an important one, and while complex boils down to a ratio of proton acceptors to donors (concerning a certain zero level of protons) and it is quantified in an equation called Total Alkalinity. It is important to note here that seawater is not just water with salt, it is a “soup” of ions and molecules.
Total alkalinity is the very complicated-looking equation below (I warned you that this was the complex answer!):
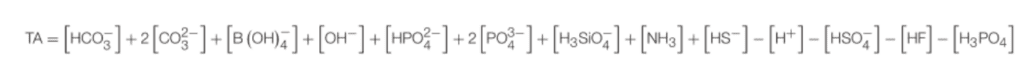
So when CO2 dissolves naturally into the ocean (which absorbs around 40% of our CO2 emissions), it causes acidification. CO2 simply dissolved in water is Carbonic Acid, hence the reason we have ocean acidification.
The dissolution of magnesium/calcium cations containing minerals leads to the consumption of H+, which is then replaced by the positively charged Mg2+/Ca2+ cations.
The positive charges from the Mg2+/Ca2+ must be balanced by negative ones due to electroneutrality. This ultimately forces the shift from CO2 to HCO3- and CO2,3- and is measurable as an increase in TA (Total Alkalinity), which is where the term “ocean alkalinity enhancement” comes from.
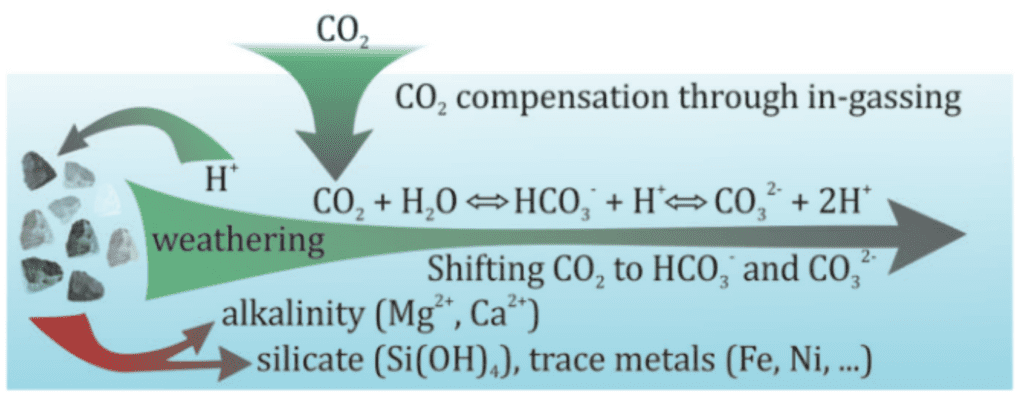
In summary, the more simple description of the complex process is:
Weathering releases magnesium ions (Mg2+) that interact in seawater (or soil pore water) to cause a reaction that “removes” carbon dioxide from the atmosphere. The addition of Mg2+ ions causes CO2 dissolved in water (carbonic acid/H2CO3) which is acidic, to undergo a chemical reaction that binds the CO2 as bicarbonate (HCO3-), which is alkaline.
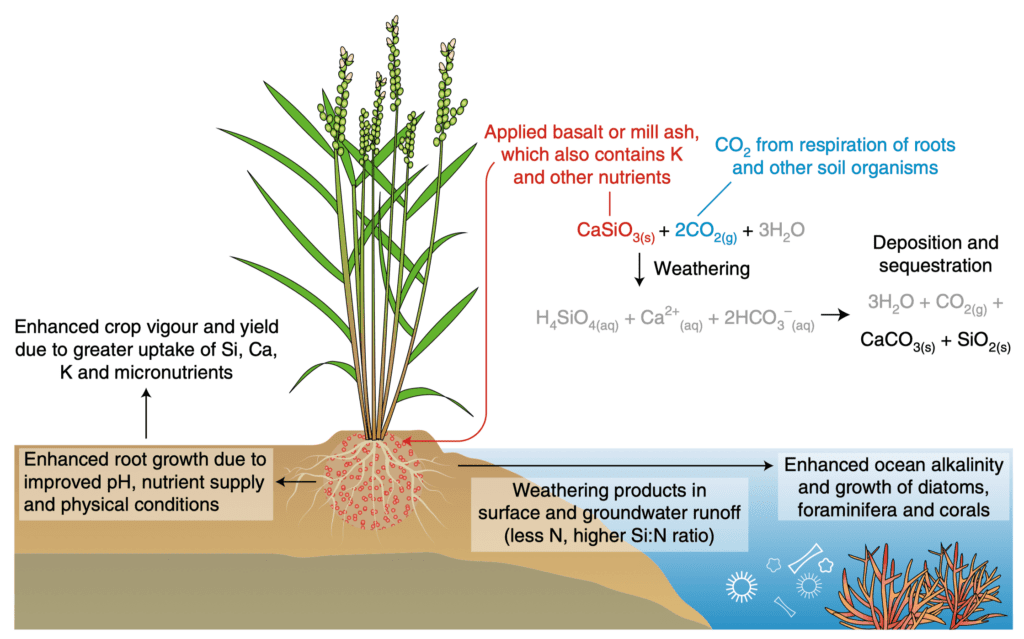
This occurs even when minerals are added on land, as the water will be transferred to the ocean and leads to the increase in Total Alkalinity (TA), “removes” carbon by “sequestering” it in bicarbonate, which stays dissolved in solution for 100,000-1,000,000 years.
Most marketplaces and buyers are considering carbon removal as permanent if it remains unreleased for over 1000 years. You’ve seen how complicated the ocean chemistry is, so we use 100,000 as our minimum for age, but it can be into the millions of years.
Sources #
CO2 Removal With Enhanced Weathering and Ocean Alkalinity Enhancement…
Farming With Crops and Rocks to Address Global Climate, Food and Soil Security
The Role of Soils in the Regulation of Ocean Acidification



